
What is the 1+MG flagship initiative?
The EU’s flagship ‘1+ Million Genomes’ (1+MG) initiative seeks to enable secure access to genomics and the corresponding clinical data across Europe. The aim is to enable groundbreaking research, health policy making, and incentivizes personalized healthcare treatments with the potential to improve disease prevention. This is one of the world’s biggest projects on genomics and contributes chiefly to setting global standards in this domain.
Genomics has become increasingly important globally. The European Union places a strong emphasis on citizen-focused and patient-friendly genomic collaboration and research. As part of this commitment, the EU ensures that the highest standards are applied to the usage, access, and storage of genomic data. The 1+ Million Genomes (1+MG) initiative, one of the world’s largest projects in this field, plays a pivotal role in setting global standards. Furthermore, its connection to the European Health Data Space will provide an additional boost to the information potential benefitting researchers, healthcare professionals and, eventually, every citizens.
Since the 2018 Digital Day, 25 EU countries, the UK and Norway signed the Member States’ declaration on stepping up efforts towards creating a European data infrastructure for genomic data and implementing common national rules enabling federated data access. The initiative forms part of the EU’s agenda for the Digital Transformation of Health and Care and is aligned with the European Health Data Space goals.
On April 10, 2018, during the 2018's Digital Day event, 25 EU countries, along with the UK and Norway, signed the Member States’ declaration aimed at strengthening efforts to establish a European data infrastructure for genomic data and implementing common national rules enabling federated data access. The initiative forms part of the EU’s agenda for the Digital Transformation of Health and Care and is aligned with the goals of the European Health Data Space.
What is the benefit for EU citizens?
Genomics has the potential to revolutionise healthcare in many ways. They can bring about the development of more targeted personalised medicines, therapies and interventions. It can also enable better diagnostics, boost prevention and make more efficient use of scarce resources. From cancer to rare diseases to neurodiseases and prevention, genomics can greatly improve health conditions of EU citizens.
Equally important, genomics has the potential to improve the effectiveness, accessibility, sustainability and resilience of health systems in the European Union.
Personalised medicine aims to offer customised prevention and treatment approaches for individual patients. Although a universally agreed-upon definition has not been established, the 2015 EU Council Conclusions outlined personalised medicine as: “A medical model using characterisation of individuals’ phenotypes and genotypes (e.g. molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention”.
What are the objectives?
The signatory countries have formulated the 1+MG main objectives:
- Ensuring that appropriate technical infrastructure is available across the EU, allowing for secure, federated access to genomic data;
- Making sure that ethical and legal implications of genomics are clear and taken into account;
- Promoting genomics awareness among the general public and policy makers in Member States and signatory countries to ensure its uptake by healthcare systems and integration into personalised healthcare.
The European Commission established the 1+MG Group - a special assembly of representatives from signatory countries - to formalize and enhance cooperation and coordination at a strategic level. This group comprises 12 specialized working groups that convene national experts to define specifications and formulate guidelines for implementing the objectives outlined in the 1+MG Declaration. Currently, the group is co-chaired by the European Commission (CNECT) and a representative from an EU country (currently Finland).
The EU as a global player in genomics
Genomics has become increasingly important globally. The European Union makes it a priority for genomic collaboration and research to be citizen-focused and patient-friendly. Therefore, it commits to ensure that the highest standards are applied for the usage, access and storage of genomic data. The EU’s 1+MG is one of world’s biggest projects of this kind and contributes chiefly to setting global standards in this domain. The connection to the European Health Data Space will provide an additional boost to the information potential benefitting researchers, healthcare professionals and all citizens, eventually.
Implementation of the Declaration: the 1+MG roadmap
The signatory countries decided to implement the 1+MG initiative along a two-staged Roadmap (2018-2022 and 2023-2027) detailing their activities across four dimensions: governance, trust framework, infrastructure and data.
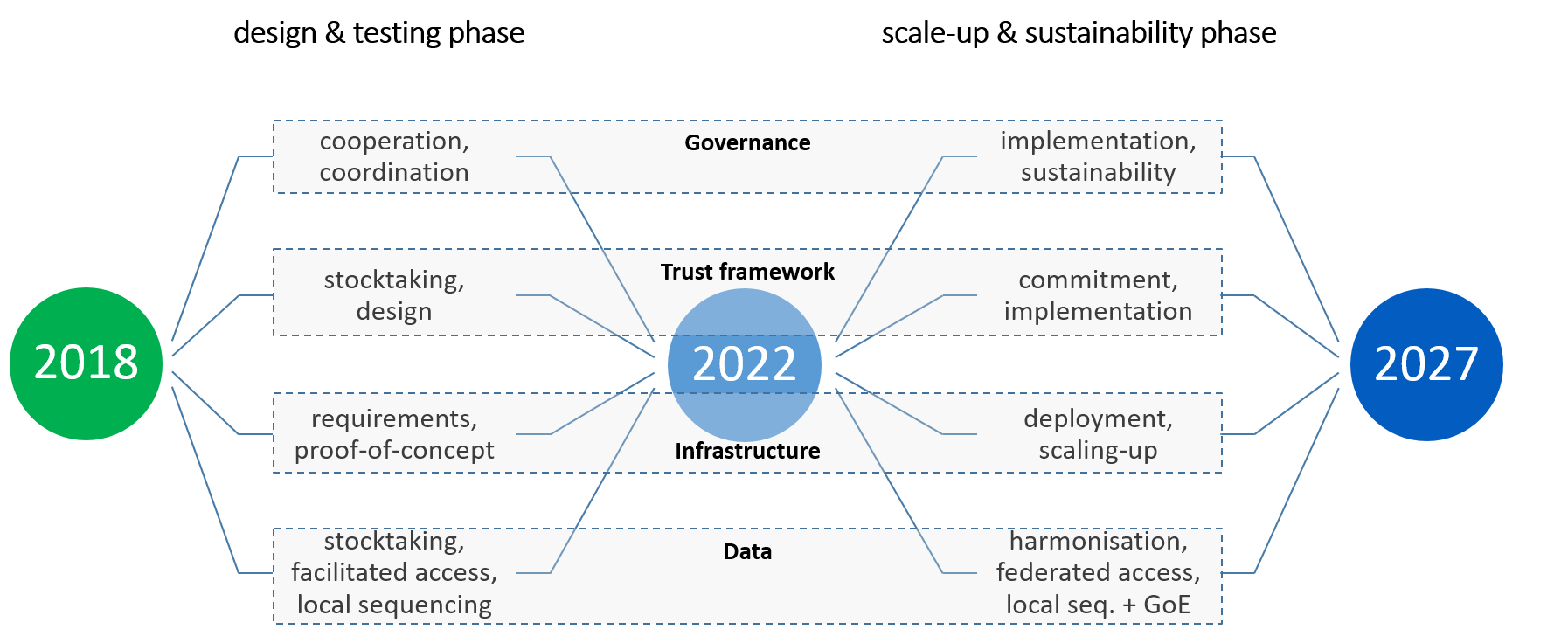
In the first phase (2018-2022), the Horizon 2020 project 'Beyond 1 Million Genomes' (B1MG) supported and coordinated the implementation of the Roadmap at operational level. During this period, several key achievements were made:
- Infrastructure set-up agreement - including technical and legal guidance, data standards, and requirements.
- Best practices and data access - facilitating data access for researchers, clinicians, and other stakeholders.
- Sustainable data sharing infrastructure - helping scientists to form a better understanding of diseases; supporting clinicians to pursue personalised medicine and driving innovators to boost the European economy. This infrastructure went beyond the immediate B1MG project goals bringing benefits for patients and enabling efficiencies in healthcare systems.
The main outcomes and deliverables of the first phase of the 1+MG initiative (see Main outcomes and deliverables section below) are presented in the 1+MG Framework website established by the B1MG project. This repository consolidates the recommendations, guidelines, and EU experts best practices, with the aim of fulfilling the Initiative’s vision of ensuring secure access to genomics and health data across Europe.
In November 2022, the Genomic Data Infrastructure (GDI) project, co-funded under the Digital Europe programme, was launched marking the beginning of the scale-up and sustainability phase of the 1+MG initiative. GDI is the main vehicle for infrastructural implementation, by establishing a decentralised data infrastructure for genomic and clinical data access across Europe. This enables distributed learning for various use cases, provides for data access governance and sustainable coordination mechanisms, but also contributes to improving the inter-operability of genomic and clinical data for access. The project is also designing and implementing a comprehensive communication strategy aiming to inform citizens and foster their trust, which is crucial for the initiative’s success.
On 14 November 2023, the 1+MG Group endorsed a roadmap (.pdf) for the second phase of the initiative (Scale-up and sustainability phase). The Roadmap 2023-2027 outlines specific activities: including implementing common recommendations and guidance, establishing the technical infrastructure, initiating infrastructure operations with research pilots in clinical use-cases, generating additional high-quality data, implementing national coordination mechanisms, and connecting the infrastructure to the EHDS and other relevant EU initiatives.
This important step is bringing Europe closer to reaching the goal of enabling secure access to genomic data across borders to advance research and personalised care in Europe. By 2024, at least six EU countries will have implemented common specifications and will be able to manage genomic data access. By 2026, 15 countries will have an operational infrastructure in place.
Genome of Europe
The Genome of Europe is a big multi-country project co-funded under the Digital Europe programme, to be launched in 2024. It will bring together European countries to build a high-quality European network of national genomic reference cohorts, representative of the European population. It will contribute to the objectives of the 1+MG Declaration and work closely with the EU-funded projects implementing it.
All countries involved will generate - via whole genome sequencing - a national genomic reference dataset based on their own national population, including both healthy and diseased individuals, according to the jointly established ‘1+MG-ready’ guidelines. Each country’s dataset will form a unique national reference collection in its own right, Collectively, cross-linked via the 1+MG Genomic Data Infrastructure, the national collections will establish a world-class European reference data resource: The Genome of Europe. This database will include data of at least 100.000 citizens, for medical research and innovation, personalised approaches in healthcare and disease prevention measures. Expected to bring major efficiencies due to economies of scale, the Genome of Europe will also ensure consistent application of the agreed common data requirements and quality measures across all national datasets.
Main outcomes and deliverables
General information:
Technical infrastructure:
- Secure cross-border data access roadmap (iterative document)
- Proof-of-concept for the rare diseases’ use case (video)
Legal, ethical and governance aspects:
- Scope of 1+MG
- Incidental findings policy
- Communication of general research results to data subjects (recommendations)
- Special (vulnerable) subjects and groups (recommendations)
Data integration and quality:
- Quality metrics for sequencing (iterative document)
- Documented best practices in sharing and linking phenotypic and genetic data (iterative document)
Healthcare implementation:
Latest news
Related Content
Big Picture
The European Commission is working to provide citizens with access to safe and top quality digital services in health and care.
Dig deeper
The 1+MG Group was created by the European Commission to support the coordination and cooperation regarding the implementation of Member States’ 2018 Declaration ‘Towards access to at least 1 million sequenced genomes in the European Union by 2022’.
See Also
The European Virtual Human Twins Initiative is an EU framework supporting the emergence and adoption of the next generation of virtual human twins solutions in health and care.
The European Cancer Imaging Initiative will unlock the power of imaging and Artificial Intelligence for the benefit of cancer patients, clinicians and researchers.
The European Innovation Partnership in Active and Healthy Ageing is an initiative that aims to foster innovation use of digital for active and healthy ageing.
The privacy code of conduct on mobile health apps aims to promote trust among users and provide a competitive advantage for those who sign up to it.
The European Commission adopted a Communication and a Staff Working Document on Digital transformation of health and care to boost European Union action.
The European Commission has adopted a Recommendation on a European electronic health record exchange format to unlock the flow of health data across borders.
The European Commission created two expert groups working on eHealth: the eHealth Stakeholder Group and a temporary eHealth Task Force.
